Ohio University chemistry and engineering faculty and graduate students authored an article on Solvent Isotopic Effects on a Surfactant Headgroup at the Air–Liquid Interface” in the Journal of Physical Chemistry C.
The authors are: Uvinduni I. Premadasa, a chemistry graduate student; Negar Moradighadi, an engineering graduate student; Kondalarao Kotturi, a chemistry graduate student; Jeeranan Nonkumwong, Md. Rubel Khan, a chemistry graduate student; Dr. Marc Singer, Associate Professor of Chemical and Biomolecular Engineering; Dr. Eric Masson, Associate Professor of Chemistry & Biochemistry; and Dr. Katherine L. A. Cimatu, Assistant Professor of Chemistry & Biochemistry.
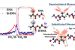
“In this study, we assess the solvent isotopic effects on the interfacial conformation of a surfactant at the air–water interface, using sum frequency generation (SFG) vibrational spectroscopy. Recent studies involving surfactants have focused on water interfacial structure or the influence of broad O–H and O–D stretch bands on the C–H stretching modes; yet, very few assess solvent effects on the structure of the interfacial surfactant itself. Here, we chose the quaternary ammonium cation 1 (see the Supporting Information for its preparation), as it belongs to the important class of “Quat” cationic surfactants used as corrosion inhibitors, especially for carbon steel in acidic media,” they write in their introduction.
Abstract: The geometry, arrangement, and orientation of a quaternary ammonium surfactant flanked by two methyl groups, a benzyl head, and an octyl tail were assessed at the air–water and air-deuterium oxide (D2O) interfaces using sum frequency generation vibrational spectroscopy. Remarkably, symmetric and asymmetric N–CH3 stretches (at ∼2979 and ∼3045 cm–1, respectively, in the SSP polarization combination) were visible in water but negligible in deuterium oxide. We concluded that D2O addition triggers the average reorientation of the dimethyl amino units parallel to the interface and possibly changes the overall conformation of the surfactant. A reduced number of gauche defects in the surfactant octyl chain is also observed in D2O. Tilt angles for the octyl chain (1.0–10.8°) are consistent with an ordered monolayer at the air–liquid interface.

















Comments