Dr. Katherine Cimatu and students in her lab recently published two papers, one on molecularly imprinted polymers with Dr. Imee Su Martinez and graduate students from the University of the Philippines – Diliman and one on substituted monomers authored solely by the Cimatu lab.
Cimatu is Associate Professor of Chemistry& Biochemistry at Ohio University and a member of the Nanoscale Quantum Phenomena Institute (NQPI).
She served as a plenary speaker in the 34th Philippine Chemistry Congress with a theme of “CHEM-Bisyon2019: Kimiko Para Sa Sariling Bayan” in May 2019 as part of her collaborative work with Martinez and as a 2019 award recipient of the Phillipine Balik Scientist Program.
Her visit boosted finishing the manuscript that has recently been published in the American Chemical Society’s Langmuir on molecularly imprinted polymers.
The hard work of her students made everything possible. Dr. Uvinduni I. Premadasa, the co-first author in the Langmuir paper, recently obtained her doctorate degree in 4.5 years, is now moving to Oak Ridge, Tenn., as a postdoctoral research fellow in the Chemical Sciences Division at Oak Ridge National Laboratory. She is graduating with seven publications (five publications as the first author) and four more manuscripts to come. Uriel Joseph Erasquin, a co-author and a third-year graduate student in her laboratory who recently passed his candidacy defense, is also from the Philippines, having graduated from the University of the Philippines – Diliman. Last, Jenna M. Berger is also a co-author and an undergraduate student researcher in her laboratory. Berger is currently the president of AXE and about to graduate this spring semester. Alpha Chi Sigma is a national fraternity for chemistry students.
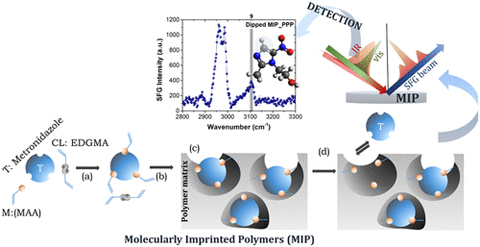
Insights on the Molecular Characteristics of Molecularly Imprinted Polymers as Monitored by Sum Frequency Generation Spectroscopy was published in Langmuir.
Abstract: Sensing in molecularly imprinted polymers (MIPs) requires specific interactions of the imprinted polymer and the approaching template molecule. These interactions are affected by the morphology of the polymer surface, the affinity of the template molecule to the polymer network, and the steric approach. In this particular study, a template molecule, metronidazole, is studied with respect to the typically used methacrylic acid-based imprinted polymer using a combination of bulk and surface techniques. The resulting infrared (IR) spectra exhibited the presence of the template molecule in the polymer matrix as well as their efficient removal after washing. Dipping of the MIP according to what is expected of facile sensing in an aqueous solution of metronidazole did not show any presence of the template molecule in the bulk of the MIP, as observed by IR spectroscopy. However, using sum frequency generation (SFG) spectroscopy, the CH aromatic stretch of the imidazole ring positioned at ∼3100 cm–1 was observed at the polymer surface, including its inner pores or cavities, and at the buried polymer-fused silica interface after dipping. SFG studies have also shown the vibrational signatures of the polymer matrix, the presence of the template molecule on the surface, and the detection of residual template molecules after washing. Increasing the washing time to 50 min has proven to be less effective than increasing the washing cycles to three. However, after the third cycle, reorganization of the polymer matrix was evident as also the complete removal of the template molecule. The observed changes from the acquired images using scanning electron microscopy and atomic force microscopy show the structural morphologies of MIPs and a good distribution of the pores across the MIP surface. The study demonstrates the importance of combining both bulk and surface characterization in providing insight into the template molecule–polymer network interactions.
Citation: Romelyn Obiles*, Uvinduni I. Premadasa*, Paul Cudia, Uriel Joseph Erasquin, Jenna M. Berger,Imee Su Martinez*, and Katherine Leslee Asetre Cimatu*, Insights on the Molecular Characteristics of Molecularly Imprinted Polymers as Monitored by Sum Frequency Generation Spectroscopy, Langmuir, 2019, DOI: 10.1021/acs.langmuir.9b02927
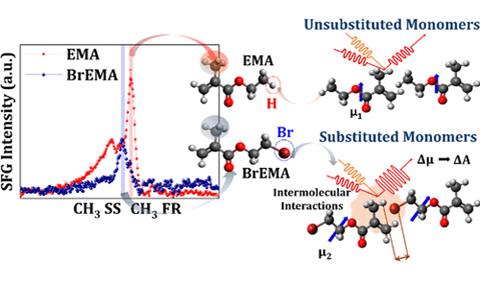
Molecular Insights into the Role of Electronic Substituents on the Chemical Environment of the −CH3 and & >C═O Groups of Neat Liquid Monomers Using Sum Frequency Generation Spectroscopy was published in the The Journal of Physical Chemistry C.
The “substituted monomers” also is authored by Premadasa. Dr. Narendra Adhikari, a co-first author in this publication, graduated in May 2019 and is now a postdoctoral research fellow at Pacific Northwest National Laboratory since June 2019. This manuscript is a part of Premadasa’s dissertation, which mainly focused on the synthesis of substituted monomers and studies existing surface and interfacial interactions using sum frequency generation spectroscopy.
Abstract: In this work, the substitution of ethyl methacrylate monomers is used as an approach to investigate the effects of electron withdrawing group substituents to their conformation at the two interfaces using sum frequency generation (SFG) spectroscopy. Cyano (−CN), hydroxyl (−OH), chloro (−Cl), and bromo (−Br) groups are substituted at the ethyl end of the methacrylate backbone to replace one H atom. Then, these neat substituted monomers are monitored at both the air–liquid (AL) and solid–liquid (SL) interfaces. The SFG spectra were recorded at different polarization combinations and infrared regions to probe specific different vibrational modes. The spectral results show relative changes in the orientation of the α-methyl (α-CH3) group with respect to variations in the substituents. This conformational change can be subsequently correlated to the carbonyl (>C═O) group, which is structurally positioned close to the α-CH3 group. The resulting intermolecular interactions in a condensed phase, especially between the α-CH3 group and the substituent in close proximity, caused spectral changes obtained at the AL interface. These spectral changes revealed variations in (1) the intensity of methyl Fermi resonance mode at ∼2935 cm–1 relative to the α-CH3 symmetric stretch, (2) the tilt angle of the α-CH3 group relative to the carbonyl group, and (3) the intensity of the C═O stretch at ∼1720 cm–1. The changes in the oscillator strengths of these vibrational modes suggested that these intermolecular interactions were triggered by the presence of these substituents in space. In addition, the overall conformation was driven by the strength and direction of the dipole moment. When Si–OH oscillator is introduced through hydrogen bonding interaction at the hydrophilic SL interface, a change in the C═O stretch SFG signal clarifies the significant contribution of the dipole moment in the changes observed at the AL interface. The key insight shows the importance of SFG spectroscopy as a tool to probe the small structural modifications of neat compounds.
Citation: Uvinduni I. Premadasa*, Narendra M. Adhikari*, and Katherine Leslee A. Cimatu, Molecular Insights into the Role of Electronic Substituents on the Chemical Environment of the −CH3 and >C═O Groups of Neat Liquid Monomers Using Sum Frequency Generation Spectroscopy, J. Phys. Chem. C, 2019, 123, 46, 28201-28209, DOI:10.1021/acs.jpcc.9b07816




















Comments