
Dr. David Drabold, Ohio University Distinguished Professor of Physics, and Bishal Bhattarai, a doctoral student in Physics & Astronomy, have published a seminal paper predicting the structure of amorphous “glassy” carbon at very low densities. Their paper provides the cornerstone of theoretical understanding of this form of matter, and is the first to accurately specify the atomic structure.
Drabold and Bhattarai show that linear “polymer” chains of carbon are part of these networks. Glassy carbon is among the hardest materials, and is a standard choice for high temperature crucibles and electrochemical applications.
Until now, there was no clear evidence that polymeric “chains” of carbon atoms existed in the solid state. Not only did Bhattarai and Drabold demonstrate the existence of such structures, they also identified their contributions to the network dynamics and the specific heat.
Their paper, Amorphous carbon at low densities: An ab initio study appears in the May 2017 issue of Carbon, an international multidisciplinary forum for communicating scientific advances in the field of carbon materials and carbon nanomaterials.
“Carbon is an endlessly fascinating element,” says Drabold. “It yields diamonds, the hardest substance, graphite, the stuff of pencils and lubricants, 60-atom soccer balls of symmetrically bonded carbon atoms, graphene, a monolayer variant of graphite, and of course carbon is the basis of all organic chemistry – and life!”
Associated with this diversity of forms, carbon atoms may bond with two, three or four companion atoms, with very similar energies in molecules. This paper reveals such bonding in the disordered solid networks. Drabold and Bhattarai computed animations to how the networks vibrate and how these motions contribute to the vibrational spectrum and specific heat.
Computing Atomic Vibrations and Their Complexity
Drabold and Bhattarai show that atomic vibrations are complex and extended through the material even displaying “mixing” between modes with different symmetry. Animations of many such modes were published with the paper.
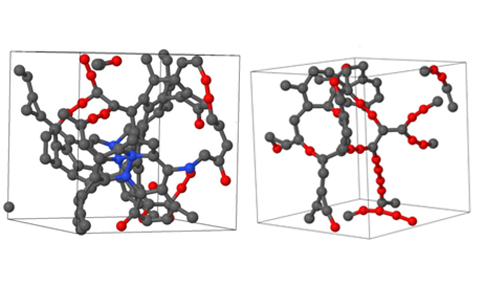
The structure of low-density amorphous carbon is modeled (left: 1.50 gm/cc, right: 0.92 gm/cc). The spheres represent carbon atoms. Red atoms have two neighbors, providing evidence of carbon chains. Grey and blue atoms have three or four neighbors, and are dominant in higher density materials.
“Our work shows that a standard assumption of this branch of materials physics is oversimplified,” explains Bhattarai. “Researchers normally think of sharply defined molecular modes involving simple motions of a few atoms. We show instead that the modes are delocalized and sometimes involve mixing between what had been thought of as distinct modes.”
Demonstrating Mixed Vibrations of Atoms with a Video Clip
Bhattarai demonstrates how the atoms vibrate in a short video clip, “Mixed vibrations—twist and twist plus stretch.”
“Two features of this video clip are noteworthy,” states Drabold. “First, there is the complexity and collective character of the modes in which all the atoms are participating in this fairly typical vibration; and second, that even in one particular mode of fixed energy, we have at least two different kinds of twisting modes displayed. This “mixing” is a feature due to the amorphous structure, unexpected from far more extensive work crystals. It would affect heat conduction and adds to our basic understanding of atomic dynamics of complex materials.”
Read their paper: ‘Amorphous carbon at low densities: An ab initio study’
Learn more about amorphous materials with Ohio University Distinguished Professor of Physics David Drabold in the 7-minute YouTube video ‘Physics of Disordered Materials’.



















Comments