Lead halide perovskites are a group of materials that are heavily being studied for a variety of applications in solar cells and light emitting diodes (LEDs). These materials have the potential to serve our demands on the green and sustainable future in energy and illumination.
However, their instability over time has jeopardized many commercial applications.
Commercially successful inorganic semiconductors are stable over time, such as silicon, the material used in computer chips and most solar panels. Similar to those in rocks, silicon atoms in the crystal lattice of the material is locked by the chemical bonds among each other, preventing them from moving.
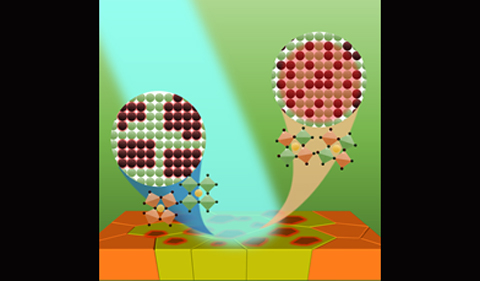
Figure. Ohio University researcher discover fast phase changes in mixed halide lead perovskite materials.
Lead halide perovskites (APbX3, the chemical symbols for the material) are ionic crystals (i.e. salts) containing lead (Pb2+, where 2+ means the lead carries two positive charges), halides (X = iodide I–, bromide Br–, and/or chloride Cl–, where the – sign means that the ion carries one negative charge), and a relatively large counter-cation (A+). These atoms are known to have measurable movements under external electric field and/or heat. It has been estimated that bromide within a perovskite lattice can diffuse ~100 nanometers per second when heated up to 200ºC. Nanometer is a unit of length, a billionth of a meter.
Ion mobility is particularly noticeable in mixed-halide lead perovskites (MHPs) where the halide composition of the crystal is a mixture of chloride, bromide, and/or iodide while the rest of the structure is the same. MHPs change color when the halide compositions change. This property has put these recipes particularly attractive to LEDs with tunable colors and tandem solar cells that absorb different colors in the solar spectrum. When the halides move, they aggregate with the same kind and cause phase segregation which creates small domains in the once uniform material. These domains have different colors that can be observed using a microscope. In the literature, the phase segregation of MHPs has been measured with ultrafast spectroscopy which measures changes below or at nanosecond level (a billionth of a second) and steady-state spectroscopy which measures changes over minutes and hours. Filling the time gap is highly desired.
Very recently, Dr. Jixin Chen, a member of the Nanoscale and Quantum Phenomena Institute (NQPI) and Assistant Professor of Chemistry & Biochemistry at Ohio University, and graduate student Juvinch R. Vicente discover surprisingly fast phase changes in MHPs within milliseconds and seconds of illumination.
Briefly, when an as-prepared chemically uniform MHP film is placed under light, the evenly distributed electrons absorb the light and start to move around. Some electrons accumulate at the defects and interfaces of the film which creates an internal electric field that drives the halides to move inside the film. Domains with different colors are immediately observed within a few seconds of illumination.
Surprisingly, these domains appear to “melt” back into the parent material over time. To explain this observation, the researchers hypothesized that the emissive domains absorb more energy and are heated up more than the film around them. As such, the aggregated ions diffuse back to the environment which “dissolves” the domains and cools down the spots. If the proposed theory is confirmed, it is important to lock the ions in the film and extra attention should be paid to the domains where the temperature can be high and degradation can be significant.
These results have been published in the Journal of Physical Chemistry Letters in an article titled “Phase Segregation and Photothermal Remixing of Mixed-Halide Lead Perovskites.”



















Comments