Ohio University’s Chemistry and Biochemistry Colloquium Series presents Dr. Michael Haley on “Phenyl-Acetylene Scaffolding as Receptors for Anions: Synthesis, Supramolecular Chemistry, and Emerging Applications” on Monday, Nov. 16, at 4:10 p.m. in Clippinger Laboratories 194.
 Haley is Professor of Organic, Organometallic, & Materials Chemistry and the Richard M. & Patricia H. Noyes Professor at the University of Oregon.
Haley is Professor of Organic, Organometallic, & Materials Chemistry and the Richard M. & Patricia H. Noyes Professor at the University of Oregon.
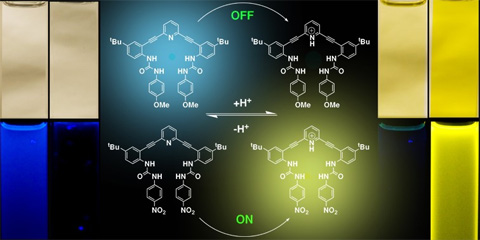
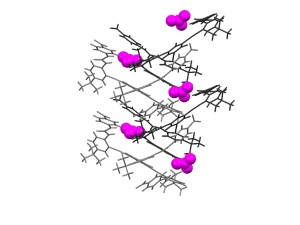
Abstract: Functionalized phenylacetylenes show broad applicability as rigid, linear subunits in the synthesis of shape-persistent supramolecular complexes. Their pi-conjugation and molecular planarity allow observation of the electronic perturbation upon formation of host-guest complexes through simple spectroscopic methods (e.g., absorption, emission). Easily substituted arenes lend tunability to the host system via bite angle, cavity size or available hydrogen bonding sites, while donor-acceptor modification enables tunability in the electronic response within each receptor class. This presentation will describe the synthesis and binding studies of sulfonamide and urea receptor complexes with a series of anionic guests, and the effect of donor-acceptor modification on their emission spectra. Emphasis will placed on utilizing these molecules as sensors for a variety of applications such as detection of environmentally relevant anions (e.g., nitrate, phosphate) and indicators of oxidative stress in vitro (e.g., redox active disulfides).


















Comments