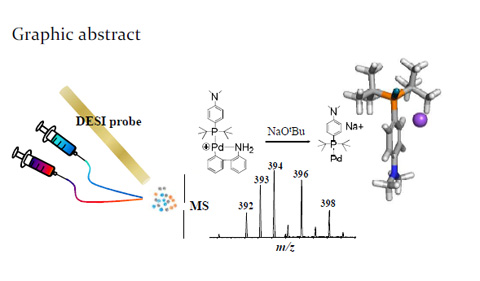
Graphic Abstract of Capture of Reactive Monophosphine Ligated
Palladium(0) Intermediates by Mass Spectrometry
Ohio University researchers and co-authors from Merck Research Laboratories authored a just-released article on “Capture of Reactive Monophosphine Ligated Palladium(0) Intermediates by Mass Spectrometry” in the Journal of the American Chemical Society.
“This study for the first time presents the detection of the reactive monophosphine ligated Pd0L intermediate by MS, which has been long regarded as the true reaction intermediates in various Pd-catalyzed coupling reactions but has heretofore not been directly observed,” says their conclusion.
Three co-authors are from the Center for Intelligent Chemical Instrumentation in the Chemistry & Biochemistry Department at Ohio University: Dr. Hao Chen, Associate Professor of Chemistry & Biochemistry with the Edison Biotechnology Institute, along with Qiuling Zheng and Mei-Hong Hu.
Additional co-authors are from Department of Process and Analytical Chemistry, Department of Structural Chemistry, at Merck Research Laboratories in New Jersey: Yong Liu, Qinghao Chen, Roy Helmy, Edward C. Sherer, Christopher J. Welch.
This work was supported by generous funding from the Merck Research Laboratories New Technologies Review & Licensing Committee (NT-RLC) and by NSF Career Award (CHE- 1149367), NSF IDBR (CHE-1455554), and NNSFC (21328502).
Abstract: A long-sought-after reactive monophosphine ligated palladium(0) intermediate Pd(0)L (L=phosphine ligand) was detected for the first time from the activation of the Buchwald precatalyst with base. The detection was enabled using desorption electrospray ionization mass spectrometry (DESI-MS) in combination with online reaction monitoring. The subsequent oxidative addition of Pd(0)L with aryl halide and coupling with amine via reductive elimination leading to new C-N bond formation were also probed using DESI-MS.



















Comments