
Dr. Katherine Cimatu in her lab in Clippinger Hall.
Dr. Katherine Cimatu, Assistant Professor of Chemistry & Biochemistry, and four graduate students in her lab published two articles in chemistry journals.
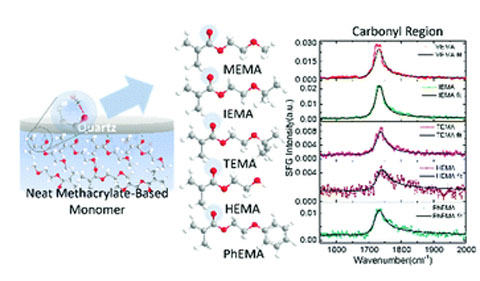
Graphical abstract: Sum frequency generation vibrational spectroscopy of methacrylate-based functional monomers at the hydrophilic solid–liquid interface
Narendra Adhikari, Uvinduni Premadasa, and Cimatu co-authored “Sum frequency generation vibrational spectroscopy of methacrylate-based functional monomers at the hydrophilic solid–liquid interface,” in the journal Physical Chemistry Chemical Physics.
Abstract: 2-Substituted ethyl methacrylate monomers were characterized using sum frequency generation vibrational spectroscopy (SFGVS) to study the effect of substituent groups in the organization of the monomers at the monomer–hydrophilic quartz interface. The SFG spectra of the methacrylate-based functional monomers collected at the hydrophilic quartz interface were found to be different from those collected at the air–monomer interface. The various spectral profiles indicated different conformations of the molecular groups at the solid–liquid interface. Moreover, a peak shift of the methyl symmetric stretch from ∼2900 cm−1 to ∼2880 cm−1 was observed from the air–liquid to solid–liquid interface, respectively. This observation of peak shifting is due to two factors: (1) a change in the chemical environment of the methacrylate-based monomer from air-to-liquid surfaces and (2) interaction between the ester carbonyl group of the monomer and the surface hydroxyl silanol group of the amorphous quartz surface. Also, the monomers were characterized in the carbonyl region, which showed the presence of the C O stretch in the SFG spectrum. This result is indicative of the hydrogen bonding interactions existing between the carbonyl group of the monomers and the Si–OH groups of the hydrophilic quartz interface. In addition, the changes in the SFG intensity of the C O peak at ∼1730 cm−1 revealed that the conformation of the C O groups is affected by bulky substituents. Furthermore, the conformational changes of these functionalized monomers at the hydrophilic solid–neat liquid interface was investigated using SFGVS.
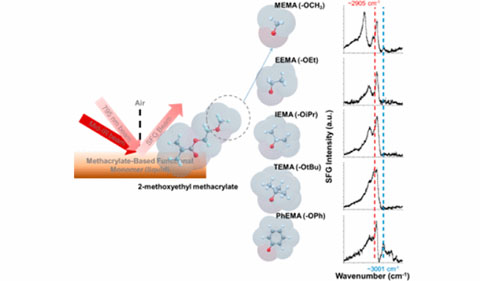
Graphic Abstract: Conformational Changes of Methacrylate-Based Monomers at the Air–Liquid Interface Due to Bulky Substituents
Premadasa, Adhikari, Susil Baral, Ahmed Aboelenen, and Cimatu co-authored “Conformational Changes of Methacrylate- Based Monomers at the Air-Liquid Interface Due to Bulky Substituents” the the Journal of Physical Chemistry. Baral is a student in Dr. Hugh Richardson’s lab, and Aboelenen is in Dr. Michael Jensen’s lab.
Abstract: Functionalization of monomers is important for various applications of polymers from electronics to surface coatings. Polymer chemists utilize methacrylate-based monomers to design polymers with wider application features. In this work, ethyl methacrylate-based monomers are functionalized with varying bulky substituents, methoxy (−OCH3), ethoxy (−OEt), isopropoxy (−OiPr), tert-butoxy (−OtBu), and phenoxy (−OPh), at the ethyl end position. These substituents are selected to understand the steric consequences of modification with a bulkier group. This study allows the determination of how the substitution affects the overall conformation of the monomer at the air–liquid interface using the sum frequency generation spectroscopic (SFGS) technique. The SFG spectral results were different for all monomers. To emphasize, the SFG intensity profile at ∼2910 cm–1 (assigned as the methyl symmetric stretch, −CH3 SS) is more noticeable in the SSP spectra compared to other vibrational modes in the SSP spectra. On the basis of the spectral and global fitting, the change in the intensity of the ∼2910 cm–1 peak was affected by an increase in the number of methyl groups in the chemical structure of the monomers. This observation has become more visible for −OiPr- and −OtBu-substituted monomers. Orientation distribution analysis was performed for the −CH3 SS of substituted −OCH3 and −OPh monomers using the calculated amplitude ratios of SSP and PPP polarizations. This analysis shows a narrow distribution angle of <30° for average tilt angles near the surface plane for the −OCH3-substituted monomer. Meanwhile, narrow distribution angles were obtained for the average tilt angles closer to the surface normal for the −OPh-substituted monomer. On the basis of the results, the change in the intensity is affected by the number density of the vibrational modes present at the interface and/or the orientation distribution of the interfacial molecules. The SFG spectral results were compared to our measured surface tension (ST) results. The ST values followed a trend with increasing number of −CH3 groups of the substituted monomers according to −OCH3 > −OEt > −OiPr > −OtBu. The −OPh-substituted monomer, 2-phenoxyethyl methacrylate (PhEMA), had a high ST value, which is the result of the intermolecular forces, including π-stacking interactions. The ST value of PhEMA favored the SFG spectral data because the aromatic CH stretch was observed and can only be detected if the −OPh group is oriented perpendicular to the surface plane. These results facilitate understanding the effects of the substituents on the conformations of the monomers. Moreover, these results will help correlate these effects with the physicochemical properties of the respective polymers.

















Comments